Abstract
Introduction The outcome of HSCT among children with chemorefractory acute myeloid leukemia (AML) is poor. The incidence of relapse exceeds 50%. We hypothesized that myeloid antigen-directed CAR-T cell therapy may induce remission in children with refractory AML.
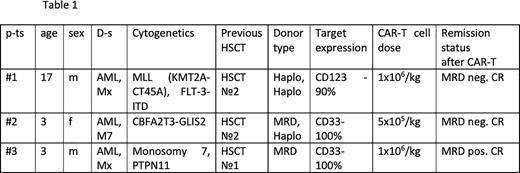
Patients and methods Three pediatric patients with R/R AML were enrolled in a compassionate-use program. All cases had AML, relapsing after multiple lines of treatment, including previous HSCT (n=3). One patient had CD123-positive leukemia cell population in the bone marrow, 2 pts had CD33-positive population. At the time of allogeneic CAR-T application disease burden was overt leukemia (n=3), all patients had full donor chimerism in the CD3 cells. Baseline characteristics of the pts are summarized in table 1.
Allo-CAR-T were derived from previous HSCT donors (haplo n=2, MRD n=1). The FlA chemotherapy regimen was used to reduce tumor burden, followed by the fludarabine and cyclophosphamide for lymphodepletion before CAR-T cell infusion in all patients. One patient received CD123 directed allo-CAR-T cells, 2 pts - CD33 CAR-T cells. Both CD33 and CD123 constructs were 2-nd generation 4-1BBz CARs, modified based on the published Ukrainskaya et al. Clinical grade manufacturing was performed on Prodigy bioreactor platform with retroviral transduction.
Results Cytokine release syndrome (CRS) occurred in 3 patients and was grade ≤3, all pts received tocilizumab with good effect. Two patients had neurologic events (ICANS grade 1-2). In one patient rash and liver toxicity coincided with CRS, potentially representing aGVHD, with full response to corticosteroids. All pts had grade III/IV hematological toxicities. The median time to CAR-T cell peak expansion was 14 days.
At 4 weeks after CAR-T infusion two patients achieved complete MRD negative remission with incomplete count recovery and good CAR-T cell expansion. One pt. had MRD positive remission with CD33 loss on residual blast cells. Two patients (#1 and #2) on d +37 and +45 receivedTotal body irradiation (TBI) at 4 Gy and TCRαβ+ depleted graft from original donors. Abatacept on d 0, +7, +14 was used as GVHD prophylaxis. One pt. (#3) received second HSCT on d +30 from haploidentical donor with full intensity TBI-based regimen. All patients engrafted recovered hematopoietic function) and had MRD-negative remission with full donors chimerism on d+30.
Two patients, #1 and #2, relapsed after 4 and 2 months after graft infusion, respectively. Patient #3 remains in MRD-negative remission on day +100. All patients alive with median follow up 164 days (102-196).
Conclusion Our early experience suggests that allogeneic myeloid antigen-directed CAR-T cells are effective as cytoreduction and have manageable toxicity. Myeloid antigen-directed CAR-T therapy can be used as a bridge to allo HSCT in r/r AML. We have documented CAR-T expansion and persistence. Prospective testing of the approach is warranted.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal